This padding only appears when menu bar covers the content below (browser width 1199px)

carlos-alfonso-HCcmfL-l08I-unsplash
We test the safety of chemicals for use in our everyday lives
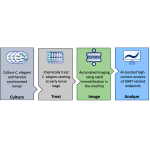
We use a C. elegans-based, New Approach Methodology (NAM) to test chemicals for systemic toxicity, developmental and reproductive toxicity, and developmental neurotoxicity. Our innovative high-throughput platform integrated with AI-enabled data analytics provides cost effective and rapid testing.
Protect public health with early identification of toxic chemicals
Help industries reduce animal use and meet regulatory compliance
Evaluate industrial chemicals that require further in vivo testing



Protect public health with early identification of toxic chemicals
Help industries reduce animal use and meet regulatory compliance
Evaluate industrial chemicals that require further in vivo testing
Latest News
Introducing vivoVerse’s Automated DART Assay: Scalable Developmental and Reproductive Toxicity Testing with C. elegans
January 30, 2026
Developmental and Reproductive Toxicity (DART) Studies Developmental and reproductive toxicity (DART) studies are crucial in identifying risks to human health ...
READ MORE →
vivoVerse revolutionizes developmental toxicity testing with AI-assisted high-throughput C. elegans image analysis
January 15, 2025
Austin, TX — vivoVerse is proud to announce the publication of groundbreaking research that sets a new benchmark for developmental ...
READ MORE →
The FDA’s Shift from Animal Testing: Role of Small Model Organisms as NAMs
April 16, 2025
On April 10, 2025, the FDA announced a major policy shift: creating a roadmap outlining a strategic approach to reduce ...
READ MORE →
NIH Pushes Human-Centered Research; vivoVerse Showcases Organ-on-a-Chip Solutions
May 7, 2025
[Austin, Texas] – The National Institutes of Health (NIH) has announced a major shift in its research priorities, emphasizing ...
READ MORE →
Unlocking the Mysteries of Protein Aggregation: Insights from C. elegans
July 31, 2025
How Dietary and Pharmacological Interventions Can Slow Progression of Neurodegenerative Diseases Protein homeostasis, or proteostasis, is essential for maintaining cellular ...
READ MORE →
More news...