![]()
How Dietary and Pharmacological Interventions Can Slow Progression of Neurodegenerative Diseases
Protein homeostasis, or proteostasis, is essential for maintaining cellular function by ensuring proper protein folding, trafficking, and degradation. Disruption of this balance can lead to the accumulation of misfolded proteins, which contributes to a wide range of disorders including neurodegenerative diseases, cancer, and age-related cellular toxicity. Detoxification systems, such as autophagy and the ubiquitin-proteasome pathway, help maintain proteostasis by removing damaged or misfolded proteins. When these systems fail, the resulting protein aggregates can become toxic.
One major group of disorders linked to impaired proteostasis is polyglutamine (polyQ) repeat expansion diseases. Huntington’s disease (HD), the most well-known among these disorders, is caused by a CAG trinucleotide repeat expansion in the HTT gene1. This mutation results in the production of a mutant huntingtin protein with an abnormally long polyQ tract, leading to misfolding, aggregation, and eventual neurodegeneration. Currently, pharmacological therapies for Huntington’s disease focus primarily on managing symptoms, but growing evidence points to dietary interventions2 as a promising avenue for slowing disease progression.
One powerful tool in this research is the nematode Caenorhabditis elegans (C. elegans), a well-established model organism3 used to study protein aggregation in the context of aging and external influences, such as diet. C. elegans provides an excellent model for studying age-related protein aggregation because of its 83% 4 genetic similarity to human-disease genes. Their transparent body allows for visualization of protein aggregates5 in real time, while short lifespan enables rapid large-scale studies.
vivoVerse’s Automated, Multi-Parametric Screening Platform for Quantifying PolyQ Aggregation in C. elegans
Traditional methods 6 to evaluate the effects of active ingredients on protein aggregation in C. elegans are a slow and costly endeavor due to manual handling of worms, individual plate preparation, and time-intensive imaging and scoring processes. This low-throughput approach significantly increases labor costs and requires extensive personnel training and time, severely limiting the number of compounds that can be tested at once. Additionally, the need to repeat experiments to ensure statistical reliability further adds to the time and financial burden. Researchers face a bottleneck in efficiently screening the vast number of compounds that could have therapeutic potential, delaying the discovery of effective interventions for Huntington’s disease.
To address this bottleneck, vivoVerse has developed a PolyQ Protein Aggregation Assay, which offers an automated and standardized method to measure how active ingredients affect protein aggregation in C. elegans. In this assay, worms that have been genetically modified to express a fluorescent reporter are exposed to a test chemical at ten different doses. Researchers then measure the ingredient’s effect on protein aggregation in aged, immobilized C. elegans using high-resolution imaging and automated analysis 7.
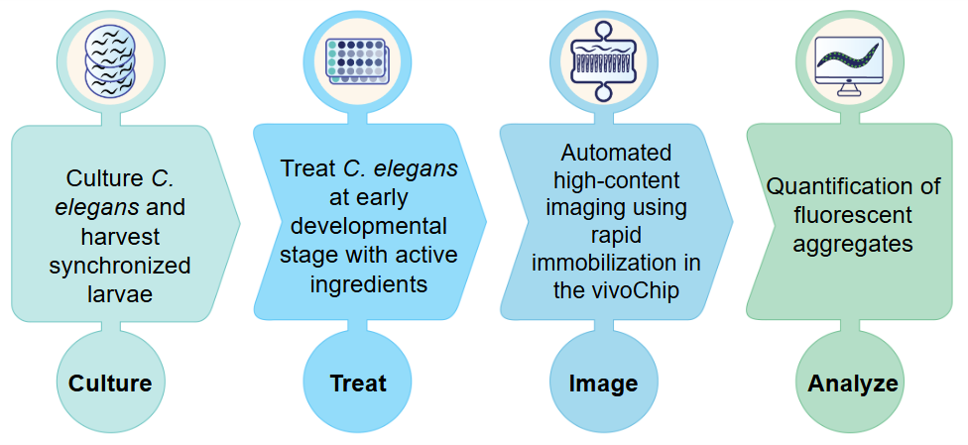
Figure 1. Schematic of an automated PolyQ Aggregate assay using C. elegans.
C. elegans strains can be genetically modified to include a green/yellow fluorescent protein (GFP/YFP) reporter, a protein that fluoresces under light when a specific gene is expressed. This allows researchers to visualize and quantify gene products in real-time. One of the strains available in vivoVerse’s assays has a YFP reporter attached to 35 glutamine repeats expressed in the body-wall muscle cells (UNC-54P::Q35::YFP). These Q35::YFP worms show a progressive transition from soluble to aggregated fluorescence signal which causes mobility loss as they age8. To assess the effect on protein aggregation of a test chemical, the C. elegans strains are treated with a range of doses at an early developmental stage. The treated worms are allowed to age before they are then immobilized in a vivoChip that captures ~1,000 worms from 24 unique populations. High-resolution brightfield and fluorescence images are acquired, and the number of fluorescent aggregates normalized per length of each animal is quantified using automated image analysis software.
The Influence of Food Ingredients on Protein Aggregation: The Need for Scalable and Precise Investigative Models
With the limited effectiveness of current drugs for neurodegenerative diseases and dementia, prioritizing prevention, delaying onset, and slowing progression has become crucial. Diet and nutrition play an important role in modulating protein aggregation9,10, though researchers are still uncovering their exact mechanisms. Certain food ingredients can either exacerbate or mitigate protein misfolding. For instance, polyphenols11—such as epigallocatechin gallate (EGCG), found in green tea—have demonstrated strong anti-aggregation properties in multiple neurodegenerative disorders. In C. elegans, EGCG was shown to reduce polyQ protein aggregation and improve motility, making it a particularly promising compound for further investigation. Other dietary compounds, including omega-3 fatty acids12 and vitamin E13, have also shown benefits in Huntington’s disease models, improving motor function and slowing disease progression. Using C. elegans, researchers can efficiently screen novel dietary compounds to determine their effects on proteostasis and identify potential nutritional interventions.
Drug Repurposing: A Large-Scale Study Identified Pharmacological Interventions that Reduced Poly-glutamine-Induced Aggregates.
In addition to dietary screening, vivoVerse’s technology has been applied to drug repurposing efforts for neurodegenerative diseases. vivoVerse’s technology7 was used to screen 983 FDA-approved clinical compounds using the PolyQ protein aggregation disease model for its relevance to Huntington’s disease in humans. Of the 983 compounds tested using the PolyQ Protein Aggregation Assay, four were found to reduce the aggregation parameters significantly – a hit rate of 0.4%. This rate of confirmed hits is comparable to the hit rate of a recent cell-based screen 14 with ~900,000 small molecules that resulted in 796 primary hits (0.09%) and 263 confirmed hits (0.03%).
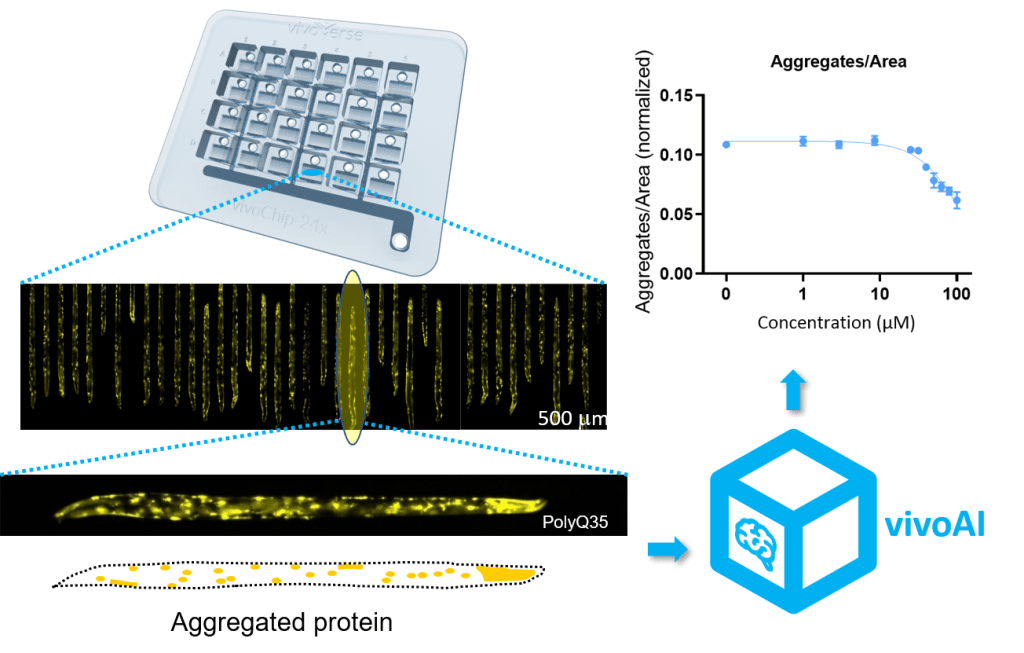
Figure 2. vivoVerse’s protein aggregation assay
Looking Ahead
C. elegans serves as an invaluable model for studying protein aggregation, particularly in relation to aging and diet. By leveraging vivoVerse’s high-content screening technologies, scientists can accelerate the discovery of compounds that influence protein aggregation, potentially leading to novel therapies for neurodegenerative diseases. Understanding the connection between aging, diet, and protein balance is an essential step toward preventing the harmful effects of protein misfolding and improving overall health. With vivoVerse’s continuous advancements in screening methodologies and computational analysis, the future looks bright for breakthroughs in treating protein aggregation disorders.
References:
-
- Caron NS, Wright GEB, Hayden MR. Huntington Disease. 1998 Oct 23 [Updated 2020 Jun 11]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2025.
- Ansari U, Nadora D, Alam M, Wen J, Asad S, Lui F. Influence of dietary patterns in the pathophysiology of Huntington’s Disease: A literature review. AIMS Neurosci. 2024 Apr 12;11(2):63-75. doi: 10.3934/Neuroscience.2024005. PMID: 38988882; PMCID: PMC11230857.
- Van Pelt KM, Truttmann MC. Caenorhabditis elegans as a model system for studying aging-associated neurodegenerative diseases. Transl Med Aging. 2020;4:60-72. doi: 10.1016/j.tma.2020.05.001. Epub 2020 Jun 10. PMID: 34327290; PMCID: PMC8317484.
- Lai CH, Chou CY, Ch’ang LY, Liu CS, Lin W. Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res. 2000 May;10(5):703-13. doi: 10.1101/gr.10.5.703. PMID: 10810093; PMCID: PMC310876.
- Zhang S, Li F, Zhou T, Wang G, Li Z. Caenorhabditis elegans as a Useful Model for Studying Aging Mutations. Front Endocrinol (Lausanne). 2020 Oct 5;11:554994. doi: 10.3389/fendo.2020.554994. PMID: 33123086; PMCID: PMC7570440.
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71-94. doi: 10.1093/genetics/77.1.71. PMID: 4366476; PMCID: PMC1213120.
- Mondal S, Hegarty E, Martin C, Gökçe SK, Ghorashian N, Ben-Yakar A. Large-scale microfluidics providing high-resolution and high-throughput screening of Caenorhabditis elegans poly-glutamine aggregation model. Nat Commun. 2016 Oct 11;7:13023. doi: 10.1038/ncomms13023. PMID: 27725672; PMCID: PMC5062571.
- Morley JF, Brignull HR, Weyers JJ, Morimoto RI. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002 Aug 6;99(16):10417-22. doi: 10.1073/pnas.152161099. Epub 2002 Jul 16. PMID: 12122205; PMCID: PMC124929.
- Xiang L, Wang Y, Liu S, Liu B, Jin X, Cao X. Targeting Protein Aggregates with Natural Products: An Optional Strategy for Neurodegenerative Diseases. Int J Mol Sci. 2023 Jul 10;24(14):11275. doi: 10.3390/ijms241411275. PMID: 37511037; PMCID: PMC10379780.
- Businaro R, Vauzour D, Sarris J, Münch G, Gyengesi E, Brogelli L and Zuzarte P (2021) Therapeutic Opportunities for Food Supplements in Neurodegenerative Disease and Depression. Front. Nutr. 8:669846. doi: 10.3389/fnut.2021.669846
- Freyssin A, Page G, Fauconneau B, Rioux Bilan A. Natural polyphenols effects on protein aggregates in Alzheimer’s and Parkinson’s prion-like diseases. Neural Regen Res. 2018 Jun;13(6):955-961. doi: 10.4103/1673-5374.233432. PMID: 29926816; PMCID: PMC6022479.
- Puri BK, Leavitt BR, Hayden MR, Ross CA, Rosenblatt A, Greenamyre JT, Hersch S, Vaddadi KS, Sword A, Horrobin DF, Manku M, Murck H. Ethyl-EPA in Huntington disease: a double-blind, randomized, placebo-controlled trial. Neurology. 2005 Jul 26;65(2):286-92. doi: 10.1212/01.wnl.0000169025.09670.6d. PMID: 16043801.
- Peyser CE, Folstein M, Chase GA, Starkstein S, Brandt J, Cockrell JR, Bylsma F, Coyle JT, McHugh PR, Folstein SE. Trial of d-alpha-tocopherol in Huntington’s disease. Am J Psychiatry. 1995 Dec;152(12):1771-5. doi: 10.1176/ajp.152.12.1771. PMID: 8526244.
- Calamini, B., Silva, M., Madoux, F. et al. Small-molecule proteostasis regulators for protein conformational diseases. Nat Chem Biol 8, 185–196 (2012).



