Developmental and Reproductive Toxicity (DART) Studies
Developmental and reproductive toxicity (DART) studies are crucial in identifying risks to human health and assessing the overall safety of chemicals and other substances present in the environment, food products and ingredients, and consumer products. Developmental toxicity studies evaluate how chemical exposures can adversely affect growth and development, while reproductive toxicity studies assess an organism’s ability to reproduce successfully1. Together, DART studies play a critical role in characterizing the safety of chemicals exposed to the public, particularly those that may affect children and pregnant women.
Numerous new chemicals with poorly characterized chronic toxicity profiles are introduced to the public every year, raising concerns over their incomplete human risk assessment. Regulatory bodies — including the Food and Drug Administration (FDA), the Environmental Protection Agency (EPA), and the Organization for Economic Co-operation and Development (OECD) — set safety standards, define toxicity parameters, and carry out risk assessments for substance approval2. These agencies also monitor adverse outcomes and restrict chemical use when necessary. For manufacturers, late-stage failures in product development pipelines or post-market attrition are costly. Hence, conducting comprehensive toxicity studies and establishing safe dosage ranges early is essential to ensure regulatory compliance and avoid costly setbacks across industries.
Traditionally, scientists have relied on mammalian models such as rats, minipigs, dogs, and non-human primates to predict human DART responses because these animals share key physiological traits with humans. However, these studies are expensive, labor-intensive, and raise ethical concerns, which have limited the number of chemicals that can realistically be tested. As a result, a significant knowledge gap remains in our ability to predict human risk. To bridge this gap and enable comprehensive toxicity profiling for the thousands of potentially hazardous chemicals in use today, researchers are turning to predictive, scalable, and non-animal platforms for DART testing. In support of this effort, the FDA has announced plans to phase out animal testing in favor of New Approach Methodologies (NAMs). Because no single test can fully capture human toxicological outcomes, the most reliable strategies combine multiple NAMs, including human cell-based systems, small model organisms, and in silico models, to achieve the most reliable results. Amongst these NAMs, the small model organism C. elegans has emerged as a particularly promising alternative thanks to its intact reproductive organs, conserved toxicity pathways, genetic similarities to humans, and ease of culture.
C. elegans as a Model for DART Studies
The nematode C. elegans has become a widely used model organism in predictive toxicology. Its rapid life cycle, prolific reproduction, and small size make it accessible and cost-efficient for laboratory studies. The relevance and predictive power of C. elegans have been well established through its fully sequenced genome, mapped connectome, traced cell lineage, and numerous genetic and signaling pathways, 60–80% of which are conserved in humans3,4,5. C. elegans is especially well-suited for DART studies because it possesses a fully functional reproductive system, active metabolism, and a transparent body that enables real-time visualization of embryo development. Its reproductive system — composed of germline cells (sperm and eggs), supportive somatic gonadal cells, and an egg-laying apparatus — shares key functional features with humans and is sensitive to environmental disruption6. The C. elegans germline has been used to evaluate aneuploidy-inducing endocrine-disrupting chemicals and has shown responses that closely reflect those observed in humans, underscoring its relevance for human risk prediction.
The vivoVerse C. elegans DART Assay
To support the growing needs of the industry, vivoVerse has developed a fully automated DART assay using C. elegans. This assay evaluates chemical toxicity by analyzing key developmental and reproductive endpoints in adult C. elegans after chronic exposure to chemicals. Age-synchronized worms are exposed to a reference chemical for 72 hours, starting from the L1 larval stage until adulthood. After exposure, treated worms are loaded into the vivoChip-24x, a microfluidic device that immobilizes ~1,000 worms across 24 distinct populations. High-resolution images are then taken for each worm, and metrics such as body length, surface area, and volume are quantified. These measurements are analyzed by vivoVerse’s machine-learning (ML) powered image-analysis pipeline, enabling rapid, scalable, and objective assessment of chemical toxicity8 (Figure 1).
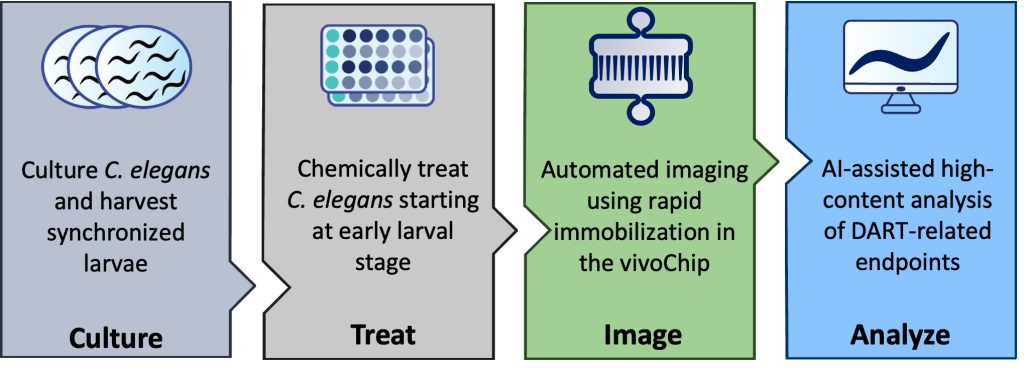
Figure 1: DART workflow. Age-synchronized C. elegans are exposed to chemicals in liquid media for 72 hours. Day 1 adults are then loaded into the patented vivoChip microfluidic device, where they are immobilized and imaged. An AI model analyzes vivoChip images to quantify multiple DART-related endpoints.
Conventionally, fecundity and brood size of gravid worms are measured using a lengthy and labor-intensive method that involves manually transferring and counting the number of embryos laid by adult worms and tracking how many of those embryos successfully hatch into larvae daily for 5 days9. In contrast, vivoVerse has developed a patented microfluidic and AI-based approach for rapid acquisition and analysis of whole-body brightfield images, enabling quantification of all in utero embryos in under 24 hours. By focusing on in utero embryos, this method avoids off-target effects associated with neuromuscular junction defects and egg-laying abnormalities. In addition, the AI model classifies each embryo by developmental stage, indicating whether it can develop beyond the 2-fold embryo stage10 (Figure 2).

Figure 2: C. elegans in utero embryo development. Brightfield 10× images of immobilized worms are analyzed using the AI-based image analysis pipeline to quantify developmental endpoints (such as body size) and embryo phenotypes. All embryos present in the parents’ uteri are classified as early-stage (magenta) or late-stage (green) embryos.
The vivoVerse AI-based image-analysis pipeline streamlines DART data analysis by eliminating the need for manual quantification of DART-related endpoints. Tasks that once required scientists to manually analyze thousands of individual worms over several days can now be completed by the AI model in a fraction of the time, reducing human error and fatigue while improving accuracy and reproducibility.
Multiple-Concentration DART Data Collection
Using the vivoChip, up to 1,400 C. elegans from 12 populations (with controls and 3 biological replicates) are analyzed across 10 concentrations. This design enables robust estimation of key toxicological metrics, including effective concentration (EC50), the lowest observable adverse effect level (LOAEL), and no observable adverse effect level (NOAEL), with strong statistical power. Manual analysis of such large datasets is impractical; however, vivoVerse’s AI-based model processes all 1,400 C. elegans in under 15 minutes. The system automatically quantifies key developmental endpoints—body length, area, and volume—and reproductive endpoints—embryo count by developmental stage—all of which are analogous to commonly used mammalian metrics. This robust assay’s automated workflow minimizes variability, achieving a maximum coefficient of variance (CV) < 8% for developmental endpoints and < 16% for reproductive endpoints, enabling efficient generation of reliable and reproducible DART data.
Case Study: DART Study of a Reference Chemical
Using the vivoVerse DART assay, we have tested several reference chemicals for DART toxicity, including agrichemicals, industrial chemicals, pharmaceuticals, and food ingredients. Figure 3 highlights the DART results of one such reference chemical. To identify toxic concentration ranges, we first conducted a range-finding study spanning from very low to very high concentrations to establish the lethal concentration window. With that information, we narrowed the dose range, created a dose-response DART curve, and quantified each DART parameter at increasing chemical exposures.
From the dose–response curve, we determined the EC50 (the concentration causing effects in 50% of the population). For this specific reference chemical, we observed the EC50 value for late-stage embryos (1.47 µM) to be 5.7x fold lower than that of the body length (8.32 µM), a commonly used developmental endpoint. Importantly, all C. elegans remained viable at all tested concentrations that produced measurable reductions in DART-related endpoints, indicating that the observed effects represent sublethal developmental and reproductive toxicity rather than general organismal lethality.
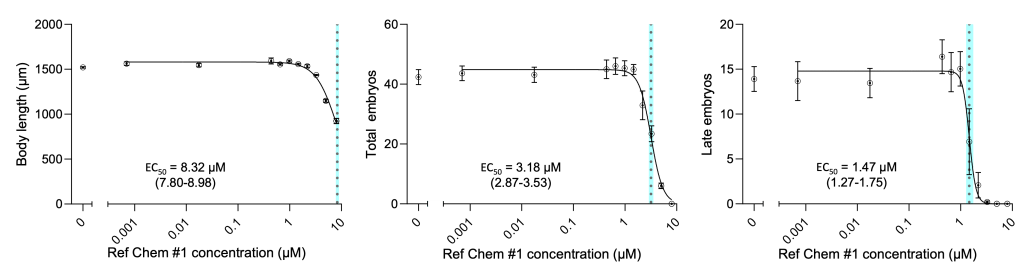
Figure 3: Concentration response curve of DART-related endpoints. The effects of reference chemical #1 on the body length (A), total embryos (B), and late-stage embryos (C). The data are represented as mean ± SEM, n = 3 biological replicates. The solid line is a 4-parameter logistic curve fit. The dashed line and blue region represent the EC50 and the 95% confidence interval, respectively.
In summary, vivoVerse delivers a next-generation platform for DART testing by uniting the biological relevance of C. elegans with automated microfluidic and AI/ML analytics. This scalable, cost-effective, and ethically responsible approach enables rapid and reproducible evaluation of chemical safety at a depth and throughput not achievable with conventional methods. By generating decision-ready, reproducible insights early in development, vivoVerse empowers researchers, manufacturers, and regulators to make faster, more informed choices while reducing reliance on animal testing and delivering actionable insights for chemical safety assessment.
References:
- Hougaard KS. Next generation reproductive and developmental toxicology: crosstalk into the future. Front Toxicol. 2021 Mar 18;3:652571.doi: 10.3389/ftox.2021.652571
- OECD. Test No. 421: Reproduction/Developmental Toxicity Screening Test, OECD Guidelines for the Testing of Chemicals. (OECD, 2016)
- Riddle D.L., et al., editors. elegans II. 2nd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1997. Section I, The Biological Model. Available from: https://www.ncbi.nlm.nih.gov/books/NBK20086/
- Kaletta, T. & Hengartner, M. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov 5, 387–399 (2006). Doi: 10.1038/nrd2031
- Hunt PR. The C. elegans model in toxicity testing. J Appl Toxicol. 2017 Jan;37(1):50-59. doi: 10.1002/jat.3357
- Athar F., Templeman N.M. C. elegans as a model organism to study female reproductive health. Comp Biochem Physiol A Mol Integr Physiol. 2022 Apr;266:111152. doi: 10.1016/j.cbpa.2022.111152
- Allard P, Kleinstreuer NC, Knudsen TB, Colaiácovo MP. A elegans screening platform for the rapid assessment of chemical disruption of germline function. Environ Health Perspect. 2013 Jun;121(6):717-24. doi: 10.1289/ehp.1206301
- DuPlissis A., et al. Machine learning-based analysis of microfluidic device immobilized C. elegans for automated developmental toxicity testing. Sci. Rep. 2025 Jan 2;15(1):15. doi: 0.1038/s41598-024-84842-x
- Ciosk, R., and B. Bowerman. “Measuring Embryonic Viability and Brood Size in Caenorhabditis elegans.” Journal of Visualized Experiments, no. 162, 2020, doi: 3791/65064
- Hall, D.H., Herndon, L.A. and Altun, Z. Introduction to elegans Embryo Anatomy. InWormAtlas. doi:10.3908/wormatlas.4.1



