Publication
Erik Lundquist Lab, University of Kansas
This study by the Lundquist lab at the University of Kansas explored the genetic regulatory pathway regulated by a Hox gene called MAB-5/HOX in Q neuroblast migration using the vivoChip platform in C. elegans. The study is the first one to show that a neuroblast is capable of instantaneously and prematurely differentiating into a neuron upon failure to migrate through in vivo imaging of a whole animal (see 60 and 120-minute time point micrographs for WT and EPHRIN-4 mutant).

Dendritic growth cone development in wild-type C. elegans

Dendritic growth cone development in ephrin-4 mutant C. elegans
“This project could not have been possible without the use of the vivoChip and so much help from you guys.”
Vedant Jain
Erik Lundquist Lab, University of Kansas
Publication
Lili Tang Lab, University of Georgia
Seth Currie from the Tang lab at University of Georgia used the vivoChip to investigate the effects of PFAS on neurodevelopment in their recent paper "Utilization of Artificial Intelligence Coupled with a High-Throughput, High-Content Platform in the Exploration of Neurodevelopmental Toxicity of Individual and Combined PFAS". They combined vivoChip technology with the Cytation 5 automated microscope and deep-learning image analysis to build a high-throughput, high-content pipeline for neurotoxicology studies.
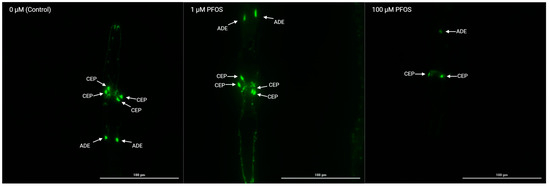
Here they show the effects of PFOS on BZ555 (dat-1p::GFP) C. elegans after 48 h of exposure. vivoChips enabled them to rapidly immobilize and capture high-resolution imaging of multiple populations of animals treated with different concentrations of PFAS chemicals and their mixtures, a feat not practical with traditional immobilization and imaging methods at this scale.
Publication
Clifford Luke, Washington University in St. Louis
Clifford Luke at Washington University School of Medicine in St. Louis used vivoChip to investigate regulation of cell survival vs death in the face of physiological stress in their recent paper "Lysoptosis is an evolutionarily conserved cell death pathway moderated by intracellular serpins" published in Nature Communications Biology.

vivoChip was used to conduct time-lapse imaging of lysosomal membrane permeabilization (LMP) as indicated by loss of fluorescent dextran. Worms null for the cysteine protease inhibitor, srp-6 showed a significant enhancement of lysosomal permeability upon hypotonic stress. The vivoChip allowed high-resolution 3D confocal imaging of the same animals every 15 m over 4 hours.
Publication
Pierce-Shimomura Lab, University of Texas at Austin
vivoChip was recently used by Wisath Sae-Lee from the Jon Pierce laboratory at the University of Texas at Austin in their recent paper "APP-Induced Patterned Neurodegeneration Is Exacerbated by APOE4 in Caenorhabditis elegans" published in G3.
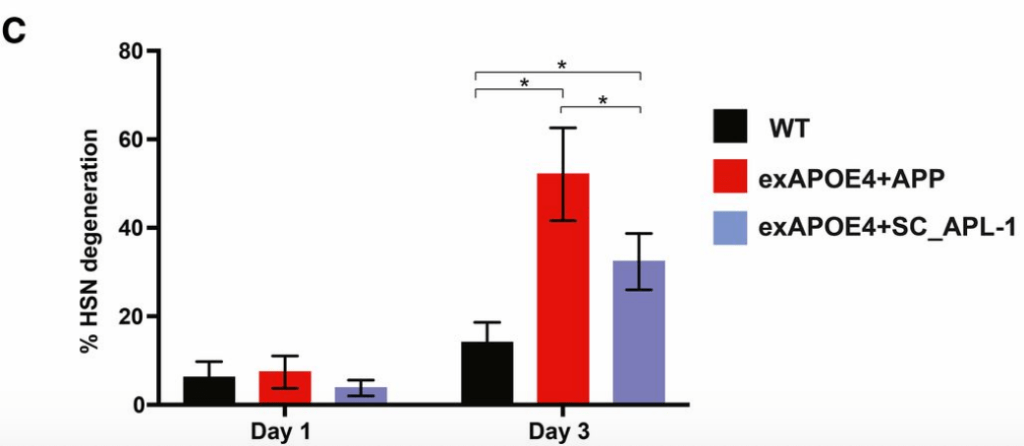
vivoChip was used to score HSN neurodegeneration and found it increased when APOE4 was overexpressed with human APP, but not a related C. elegans protein APL-1. It suggests interaction between APP and APOE4 is important in the development of Alzheimer's
"vivoVerse deliver on their promises, and have enabled our lab to perform high-throughput imaging in C. elegans. Their system is very easy to set up and use, the worms are very robustly oriented, and the chip is suitable for even high-resolution imaging!"
Steven Ban,
Saul Kato Lab, UCSF

NeuroPAL C. elegans (polychromatic, multi-neuronal marker gene) in a vivoChip. Imaging conditions: 40X, 1.3NA, volumetric image taken over 50µm, maximum intensity projected. Image courtesy of Steven Ban, UCSF
“We find that vivoChip is a very intelligent system, easy to use and very fast. It is really impressive, and we were so happy to see these beautiful pictures that we took using wide-field and spinning disc microscopes.”
Claudia Maios and Gilles Tossing
Alex Parker Lab, University of Montreal
Dr. Parker’s lab members are using the vivoVerse vivoChip platform to immobilize age-synchronized adult animals in the device. Gilles was able to visualize up to 9 days adult C. elegans expressing fluorescent markers and interested to use the platform for long-term imaging experiments.

Fluorescence images of unc-47::mCherry animals immobilized inside the vivoChip. Widefield and spinning disc images of 8 days adult animals were captured to score for individual neurons and their connections in a high-resolution time-lapse experiment. Image courtesy of Gilles Tossing, CHUM.
“Day 2 of using the vivoCube from vivoVerse ! Still learning some tricks to improve/perfect the set-up process, but super excited to do science with this new toy! Huge thanks to vivoVerse support for getting us started.”
Joseph Liang,
Catharine Rankin lab, University of British Columbia

pdr-1(tm1598), pdat1::GFP C. elegans (D1). These are a part of an imaging experiment that investigates whether there is more neurodegeneration as these worms age. pdr-1 is the worm homolog for human PRKN, a gene implicated in Parkinson’s Disease. Image courtesy of Joseph Liang, UBC
“The vivoCube and vivoChip system has revolutionized my imaging days! It’s easy to set up, fits nicely into our space, and is very user-friendly. And it’s so fast! I can image and score a full chip in about 40 minutes!”
Lotti Brose,
Pierce-Shimomura Lab, University of Texas at Austin

Also from the Jon Pierce laboratory at the University of Texas at Austin is Dr. Lotti Brose. She has been using the vivoVerse (formerly Newormics) D1-D5 vivoChips to study HSN neurons (part of the C. elegans egg laying circuitry) in D1-D3 animals. Lotti has been particularly happy with the speed at which worms can be imaged, and is able to reuse her vivoChips multiple times for different batches of worms.
“I found the L4-YA chip from vivoVerse extremely effective in immobilizing L4s and adults for live imaging even without supplementing the system with any anesthetics. The imaged worms also seemed to be in good condition when the imaging session was concluded (approximately one hour), which is great in cases where the worms need to be recovered for propagation.”
Nikhil Mishra,
Barbara Conradt Lab, LMU Munich
vivoVerse Customers include

























